|
|
|
|
|
Research Interests:
Single Molecule Biophysical Chemistry Development and Applications of Nanocrystals for
Biophysical Applications Spectroscopic Properties of Single Nanoparticles Novel Techniques Applied to Single Molecule Research Single Molecule Biophysical
Chemistry Most
biochemical processes are complex and intrinsically heterogeneous. Biomolecules
adopt a wide range of conformations to perform their specific functions.
These conformations are constantly changing with time and environment. We are
studying these changing conformations to more thoroughly understanding
biological mechanisms. Single molecule
fluorescence allows us to study the heterogeneity of the various
conformations, as well as quantifying the timescales of the conformational
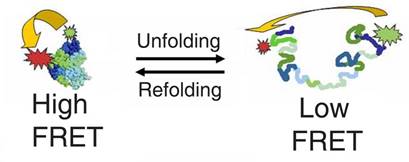
transitions by using Fluorescence Resonance Energy Transfer (FRET). FRET uses
the fact that fluorophores can transfer energy when they are in close proximity, and the efficiency of this transfer is
extremely
sensitive to distance - therefore it can be used as a ruler to measure very
small distances (1 - 10 nm) between fluorophores that are placed at specific
sites. With our microscope, these distances can be measured on a single
molecule as a function of time, so, as a biomolecule changes conformation,
the distance between the fluorophores change, and we can determine how long
these transitions take, and how many different conformations are present. The
great thing about doing this at the single molecule is that we see the
transitions directly - we don’t need a mathematical model to extract the
data. Also, we can do the experiments under equilibrium since, at the single
molecule level, molecules are constantly fluctuating between conformations.
We don’t need a ‘trigger’ to start the process. This technique
is highlighted in the figure below, which allowed us to follow the
folding-unfolding pathway of a protein (For more details on the protein
folding work, see our publications).
We are now using such
techniques to address important Health and Energy issues, specifically
cancer, neurological disorders and plant photosynthesis. (A)
protein interactions related to the onset of cancer (proteins known as growth
factors) and how anti-cancer drugs interact with their targets. Small (often
benign) tumors need nutrients in order to grow into larger, more dangerous
tumors. They get these nutrients by forming blood vessels which then connect
to the blood supply. This process is called Angiogenesis and, when regulated, is critical to allow new
healthy cells to grow. Cancer cells can break down this regulation, allowing
them to grow faster than normal cells, which can then lead to tumor growth
and eventually metastasis. Angiogenesis is
initiated by a growth factor interacting with a growth factor receptor, which
starts a complicated signaling process in the cell. By regulating this
interaction, either naturally (autoregulation) or with drugs, angiogenesis
may be controlled. This may be an effective way of treating cancers at very
early stages. If we are to do this, we need to understand the process of
angiogenesis and how to improve drug interaction with these potential
targets. The particular proteins that we are studying are fibroblast growth factor (FGF)
interacting with its receptor (FGFR) at the single molecule level. What
factors regulate the strength of this interaction and what are the underlying
protein dynamics involved. (B)
Structure of neurological receptors (glutamate and glycine receptors) in live
cells and organisms. Many neurological
disorders can be related to the improper recognition of neurotransmitters
such as glycine and glutamate by their receptors. This recognition is highly
dependent of the structure and arrangement of the various subunits that make
up the receptor, and is highly variable depending on the function, organism
and environment. We are elucidating the structure-arrangement function
relationships between these various glycine and glutamate receptors in living
cells and even living organisms using our single molecule fluorescence
techniques. We recently published a paper on using specific subunit
labeling by genetically engineering them with green fluorescent protein (GFP)
and used single molecule fluorescence together with stepwise photobleaching
of the GFP to literally count the
number of different types of subunits present in the human glycine receptor
expressed in Xenopus Oocytes. (C)
Mechanisms of how plants transport and organize the proteins involved in
photosynthesis. Photosynthesis
in plants requires the specific organization of proteins that bind
chlorophyll in the thylakoid membrane - imaginatively called light-harvesting chlorophyll-binding
proteins (LHCP). This specific organization of proteins is called the light harvesting complex, and allows
photosynthesis to be very efficient at focusing the light energy to a
reaction center so that photosynthesis can occur - by which the plant
produces chemical energy from solar energy. If we can learn how plants
organize these proteins in such a way, we can perhaps learn how to mimic the
process for our own needs in renewable solar energy. We may even be able to
use the plant targeting proteins themselves to arrange light harvesting
complexes for us. We use our single molecule fluorescence techniques to study
how plants use multiple proteins (at least 5 of them) to target the LHCP into
the thylakoid membrane and then insert it at the correct time and place. Development and Applications of
Nanocrystals for Biophysical Applications One of the
limitations of single molecule fluorescence experiments is that high
excitation powers are necessary to detect the fluorescence from a single
molecule. This means that the fluorophore will spend a lot of time in an
excited state. When in this excited state, oxygen or other chemicals may
react with it and cause it to become non-fluorescent. This process is called
photobleaching, and organic fluorescent dyes suffer from it significantly, and
it limits the maximum time that a single molecule can be observed. We are
overcoming this limitation by using fluorescent inorganic nanocrystals, which
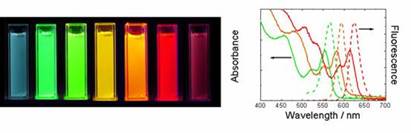
are also called quantum dots (QDs). These nanoparticles (QDs) have other
advantageous optical properties: They have a narrower fluorescence spectrum
compared to organic molecules, can be excited at any energy above their
bandgap (a result of the band structure of the energy levels) and, due to the
effects of quantum confinement, the emission spectrum (color) can be tuned
simply by changing their size. These properties are highlighted below.
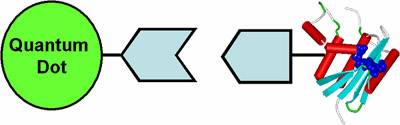
However, the
quantum dots are synthesized in organic solvents, and if we plan to use them
for labeling biomolecules, they must be made water-soluble. We are developing
techniques to enable this, which include the design of water-soluble ligands
which can bind to the surface of the quantum dot. After binding, the quantum
dots take on the chemical properties of the ligand that we have attached.
However, adding these ligands increases the overall size of the nanoparticle,
and we must be careful not to make them too large for the biomolecule that we
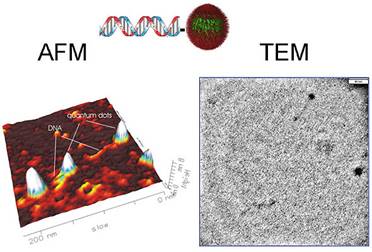
want to attach to it. The general schematic of this quantum dot-bioconjugate
is shown below.
In order to
make the conjugation reaction specific to a particular biomolecule, we vary
the connection between the quantum dot and the biomolecule depending on the
bioconjugation reaction which we want to perform. We bind the quantum dot to
proteins, DNA or lipid molecules depending on the system that we are
studying. Once the
nanoparticles are conjugated to a biomolecule, we can use the optical
properties of the nanoparticle to study the biological questions of the
biomolecule. Alternatively, we can use the biomolecule to assemble
nanoparticles into specific higher order structures. Biomolecules are able to
bind specifically to other biomolecules in well-defined geometries and
stoichiometries. One example of such a system is the strong, specific binding
of a single-stranded DNA molecule to its complementary strand to form a
helical double-stranded DNA by the use of Watson-Crick base pairing. Other
examples include protein-ligand interactions such as the strong binding of 4
biotin molecules (also known as Vitamin H or B7) to a streptavidin protein
molecule in a tetravalent, tetrahedral geometry. We are using these
biological interactions to assemble nanoparticles into pre-defined geometries.
By assembling them in such a way, the nanomaterials couple to each other, and
their optical properties are affected. We are taking advantage of this for
the next generation of optoelectronic and biosensor applications.
Spectroscopic Properties
of Single Nanoparticles Semiconductor
nanoparticles, or quantum dots, are very interesting but complicated systems.
If we want to use a single quantum dot as an optical probe for a single biomolecule,
we must also understand the spectroscopic properties of a single quantum dot.
One particular property that single quantum dots possess is a process known
as “blinking”. Upon constant illumination, the quantum dots switch “on” and
“off” – i.e. they go from “fluorescent” to “dark” – for which the mechanism
is not yet fully understood. We are investigating the underlying mechanism of
this blinking so that we can either eliminate it, or at least take it into
account, when we are interpreting our single molecule fluorescence data. An
example movie of single blinking quantum dots immobilized onto a glass slide
is shown below.
By analyzing
the timescales of this blinking process under different conditions, we can
determine which parameters affect it, and thus attempt to eliminate it. Also,
by knowing how various parameters affect blinking, we can take this into
account when we need to analyze the quantum dot-biomolecule conjugate under
certain conditions (such as inside a cell). By
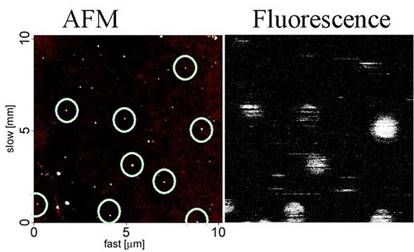
simultaneously analyzing the fluorescence image of single quantum dots and
the topography of the quantum dots using atomic force microscopy (AFM), we
have found that there are a fraction of quantum dots that are permanently
“dark” – they never fluoresce. An example of a fluorescence image and a
simultaneously measured AFM image is shown below. The fluorescent
quantum dots are circled on the AFM image, but it is clear that there are
more quantum dots physically present than the fluorescence image shows. We
are investigating the causes underlying this phenomenon using combined AFM
and fluorescence microscopy (see below).
Novel
Techniques
Applied to Single Molecule Research The following
techniques are used for our single molecule experiments:
Data are
analyzed using the following techniques:
|
|
|
|
|
|
|
|
|
|
|
Last Update: March 7th, 2018 |
|